Team building 2025

Eshmoun in celebrating by bringing together its teams from Tunisia and Morocco for a weekend in the heart of nature! 🌿
A time for sharing, reconnecting and strengthening bonds.
eshmouncompany@eshmoun.com.tn
Immeuble nour, rue du corail, Lac2 1053 Tunis
+216 27 870 563

Eshmoun in celebrating by bringing together its teams from Tunisia and Morocco for a weekend in the heart of nature! 🌿
A time for sharing, reconnecting and strengthening bonds.

Eshmoun-Clinical Research, through its Moroccan subsidiary, has signed an agreement with the Moroccan Association of Supportive Care in Cancer (MoASCC) to strengthen clinical research, clinical trials, RWE and training. Together, they are committed to working to contribute to improving the care of cancer patients in Morocco.

On the sidelines of the celebration of the International Clinical Research Day on May 20, signing of a partnership agreement between the Tunisian Society of Pediatric Oncology and the CRO Eshmoun-Clinical Research.
The agreement mainly focuses on collaboration in the field of clinical research, through the initiation of observational studies, real-life studies (RWE) as well as training programs in clinical research.

In the occasion of the celebration of the International Clinical Research Day, celebrated throughout the world on May 20 of each year, the Medical Research Direction of the Tunisian Ministry of Health, in collaboration with the National Chamber of CROs, the Ordinary Councils of Physicians and Pharmacists, the CNIP and the SEPHIRE, took an excellent initiative by organizing a day of training and exchange with the directors and administrators of public establishments participating in clinical trials. A very fruitful meeting which will certainly contribute to boosting clinical research in Tunisia.
Eshmoun focuses on cybersecurity benchmarks, implements high-level systems to comply with these benchmarks and standards, and conducts internal cybersecurity audits.
 CVCT Washington December 9-11, 2024Because health is global, clinical trials must be global and guidelines must be global. International guidelines are not international because they are based on evidence from clinical trials, mainly generated in Western-style healthcare systems. These were the main conclusions of the “Globalization and diversity in the CVCT” sessions.
CVCT Washington December 9-11, 2024Because health is global, clinical trials must be global and guidelines must be global. International guidelines are not international because they are based on evidence from clinical trials, mainly generated in Western-style healthcare systems. These were the main conclusions of the “Globalization and diversity in the CVCT” sessions.
“Cardio Vascular Clinical Trials ( CVCT) going Global” and “The CRO experience in conducting clinical trials in Africa and middle East” are the two sessions at the CVCT Forum held in Washington, during which Dr Chokri Jeribi sharedEshmoun Clinical Research ‘s vision and experience in its commitment to the promotion and development of Clinical Research (CR) in Africa in the presence of CR experts from around the world.
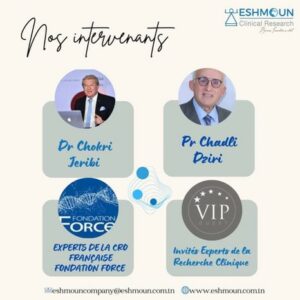 Online clinical research training : 7th class session 2024 – 2025
Online clinical research training : 7th class session 2024 – 2025
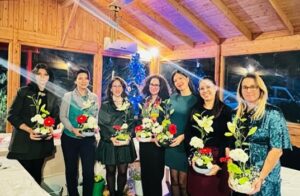 Team building To inspire, motivate and strengthen team spirit, Eshmoun regularly organizes team-building activities around meals and personal and professional development workshops, creating a close-knit, dynamic and high-performance team.
Team building To inspire, motivate and strengthen team spirit, Eshmoun regularly organizes team-building activities around meals and personal and professional development workshops, creating a close-knit, dynamic and high-performance team.
 CVCT Washington December 9-11, 2024Because health is global, clinical trials must be global and guidelines must be global. International guidelines are not international because they are based on evidence from clinical trials, mainly generated in Western-style healthcare systems. These were the main conclusions of the “Globalization and diversity in the CVCT” sessions.
CVCT Washington December 9-11, 2024Because health is global, clinical trials must be global and guidelines must be global. International guidelines are not international because they are based on evidence from clinical trials, mainly generated in Western-style healthcare systems. These were the main conclusions of the “Globalization and diversity in the CVCT” sessions.
“Cardio Vascular Clinical Trials ( CVCT) going Global” and “The CRO experience in conducting clinical trials in Africa and middle East” are the two sessions at the CVCT Forum held in Washington, during which Dr Chokri Jeribi sharedEshmoun Clinical Research ‘s vision and experience in its commitment to the promotion and development of Clinical Research (CR) in Africa in the presence of CR experts from around the world.
 November 2024 The Africa region is developing rapidly in the field of clinical research! Eshmoun is committed to building capacity by providing skilled resources to conduct clinical trials, adhering to Good Clinical Practice (GCP), national regulations and research protocols. Successful training session organized for investigators at Dakar’s main hospital on clinical research and Good Clinical Practice. Training accredited by the prestigious Instance Nationale de l’Evaluation et de L’Accréditation en Santé (National Health Evaluation and Accreditation Body)
November 2024 The Africa region is developing rapidly in the field of clinical research! Eshmoun is committed to building capacity by providing skilled resources to conduct clinical trials, adhering to Good Clinical Practice (GCP), national regulations and research protocols. Successful training session organized for investigators at Dakar’s main hospital on clinical research and Good Clinical Practice. Training accredited by the prestigious Instance Nationale de l’Evaluation et de L’Accréditation en Santé (National Health Evaluation and Accreditation Body)
